Evidence Assessment & Coverage Implications
At MA360i, we deliver comprehensive assessments of clinical evidence and coverage implications for high-impact, breakthrough diagnostic tests.
Our analysis is designed to help innovators navigate the complex landscape of payor decision-making and support evidence generation strategies that align with coverage requirements.
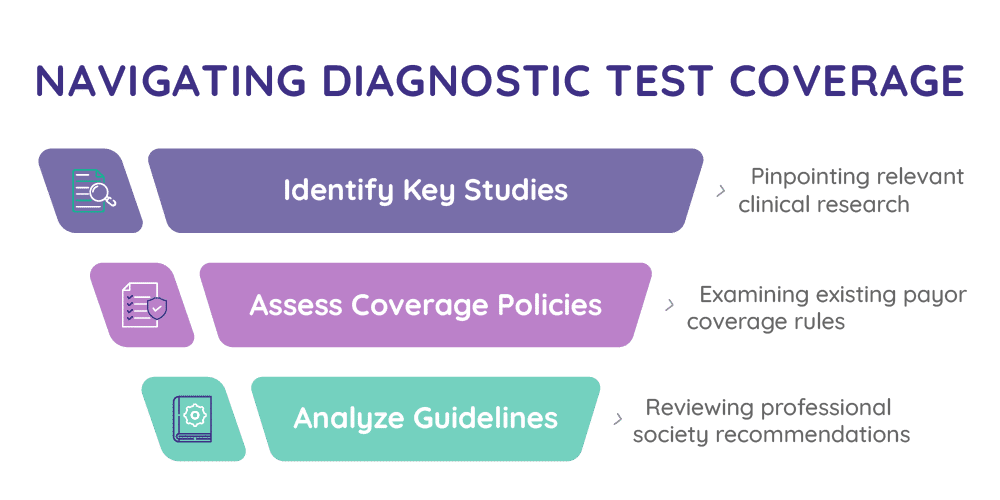
Using advanced AI-driven tools, we provide a concise and actionable summary of:
Published Clinical Studies
Professional Society Guidlines
Existing Payor Coverage Policies
This integrated analysis offers a clear view of the current evidence environment and identifies gaps that may affect payor coverage. Whether you’re preparing for payer engagement, refining your evidence development strategy, or planning market access activities, MA360i equips you with the insights needed to accelerate adoption and reimbursement of your test.
Explore our latest in-depth analysis of clinical evidence and coverage implications for Minimal Residual Disease (MRD) Testing in Solid Tumors:
Now available for purchase,
contact us to learn more.
Access the infographic
summarizing key findings.
More assessments focused on high-impact, breakthrough category diagnostics are in development and will be released soon.
Let MA360i help turn evidence into access!