Evidence Assessment & Coverage Implications
At MA360i, we deliver comprehensive assessments of clinical evidence and coverage implications for high-impact, breakthrough diagnostic tests.
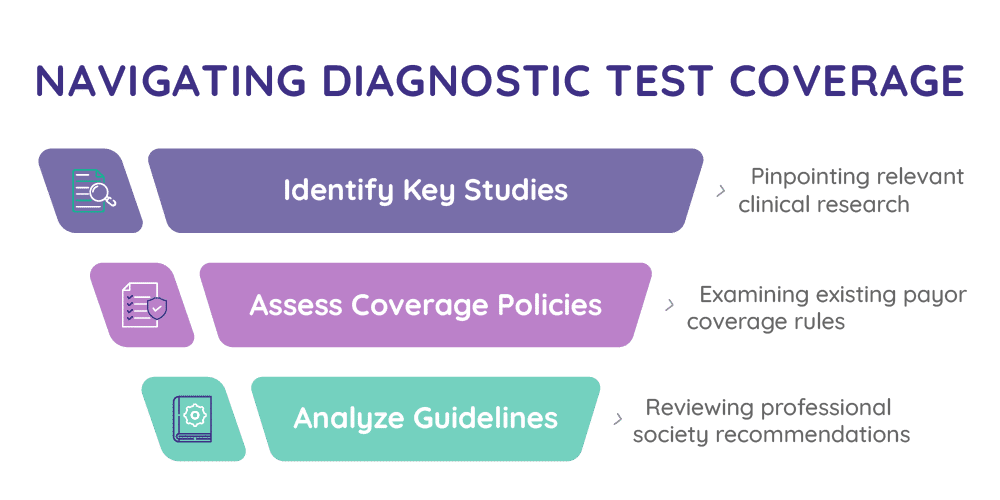
Our analysis is designed to help innovators navigate the complex landscape of payor decision-making and support evidence generation strategies that align with coverage requirements.
Evidence Generation Guidance Reports
Our reports lay out the payor perspective in a rigorous and robust way.
It includes a comprehensive view of clinical evidence, coverage analysis, payors’ perspectives on the evidence available, and future outlook.
These reports can aid test providers in planning strategic evidence development and market access efforts:
Highlights key evidence gaps and payor-identified needs to guide study design
Flags critical data points needed to close coverage gaps
Supports faster time-to-coverage and reimbursement
Clinical evidence assessment report: Multi-Cancer Early Detection/Screening (MCED/MCD)
The report lays out payor perspective in a rigorous and robust way
Clinical evidence: comprehensive review of published clinical data
Coverage analysis of existing payor policies and gaps
Strategic insights: anticipated cost savings, value of early diagnosis and potential cost shift between payor segments.
Clinical evidence assessment report: MRD Testing in Solid Tumors
The report lays out payor perspective in a rigorous and robust way
Clinical evidence: comprehensive review of published clinical data
Coverage analysis of existing payor policies and gaps
Strategic Insights: outlook on MRD testing for solid tumors.
The science got you here.
The payor-centric evidence gets you paid.